Recurrent Pericarditis
About the Cardiol 100-004 Study (MAvERIC-Pilot)
Cardiol 100-004 is a Phase 2 multi-center, open-label, pilot study assessing the impact of CardiolRx™ (cannabidiol) oral solution, on recurrent pericarditis. (ClinicalTrials.gov Identifier: NCT05494788)
Patients with recurrent pericarditis who are refractory or intolerant to current treatments or who are corticosteroid-dependent to control their disease are particularly challenging to manage clinically. Current first- and second-line management consists of non-steroidal anti-inflammatory drugs (NSAIDs), colchicine and corticosteroids. Chronic corticosteroid use has been associated with serious adverse effects and increased risk of recurrence. Third-line therapy includes anakinra and rilonacept, both of which inhibit interleukin (IL) -1 activity; however, recurrences may also occur following discontinuation of these immunosuppressive biologics.
An initial episode of pericarditis is often triggered by a viral infection or pericardial insult, resulting in aberrant activation of the inflammasome signaling pathway. This mechanism sets off a cycle of auto-inflammation within the pericardium resulting in recurrences. A small molecule drug with a favorable safety profile that attenuates the upstream intracellular inflammasome signaling processes leading to the release of pro-inflammatory interleukins, represents a novel approach for the treatment of recurrent pericarditis.
The MAvERIC-Pilot study will investigate the safety and efficacy of CardiolRx™ administered over 8 weeks in adult patients with at least two prior episodes of recurrent pericarditis. There will also be an 18-week extension period to assess the feasibility of weaning concomitant background therapy, including corticosteroids, while taking CardiolRx™.
What is the Investigational Product in the Cardiol 100-004 Study?
CardiolRx™ is a pharmaceutically manufactured cannabidiol solution, formulated for oral administration. Cannabidiol is known to have anti-inflammatory properties.
Although the mechanisms of CardiolRx™’s anti-inflammatory effects are not fully understood, research suggests that the drug substance in CardiolRx™ attenuates multiple inflammatory signaling pathways, including inhibiting activation of the NLRP3 inflammasome.
Pericardial insult or viral infection results in aberrant expression and activation of the NLRP3 inflammasome protein components which induce the release of pro-inflammatory cytokines (e.g., IL-1α, IL-1β, IL-6, & IL-18). This pro-inflammatory cytokine release perpetuates endothelial dysfunction, impairs vasodilation, and activates leukocytes, thereby leading to pericardial damage, increased pericardial space and thickness, and a cyclic release of IL-1α.
The drug substance in CardiolRx™ has been shown to significantly decrease the pro-inflammatory cytokines IL-1β and IL-6 and inhibit pro-IL-1β and NLRP3 mRNA expression in vitro, and significantly reduce pericardial effusion and thickness in a murine model of acute pericarditis.
NLRP3 = NACHT, leucine-rich repeat, and pyrin domain-containing protein 3
What are the Study Objectives?
Efficacy Objectives:
The primary efficacy objective is to evaluate the effect of CardiolRx™ on patient-reported pericarditis pain score using an 11-point Numerical Rating Scale (NRS), following 8 weeks of treatment.
Additional efficacy parameters include:
- Change in C-reactive protein (CRP) level
- Time to normalization of CRP level (if abnormal at baseline)
- The percentage of patients with a pericarditis recurrence during an extension period
The study will also assess the feasibility of weaning concomitant pericarditis treatments, including corticosteroids, while taking CardiolRx™.
Safety Objectives:
The primary safety objective is to demonstrate that administration of CardiolRx™ in the proposed doses in this patient population is safe, as determined by measuring several parameters, including adverse events and changes in ECG and blood laboratory tests.
Who is Eligible for the Study?
Key Inclusion Criteria:
- Male or female patients aged ≥18 years
- Diagnosis of at least 2 episodes of recurrent pericarditis
- At least 1 day with pericarditis pain score ≥4 on the 11-point NRS within the prior 7 days
- C-reactive protein level ≥1.0 mg/dL OR evidence of pericardial inflammation assessed by delayed pericardial hyperenhancement on cardiac magnetic resonance imaging
- Currently receiving non-steroidal anti-inflammatory drugs (NSAIDs), colchicine or corticosteroids for treatment of pericarditis (in any combination) in stable doses
Key Exclusion Criteria:
- Diagnosis of pericarditis secondary to the following etiologies:
- Tuberculosis
- Neoplastic, purulent or radiation etiology
- Post-thoracic blunt trauma
- Myocarditis
- Prior history of sustained ventricular arrhythmias or QT interval prolongation
- Taken any cannabinoid in the past month
- Current diagnosis of cancer (except for non-melanoma skin cancer)
- Immunosuppressive therapy with any of the following treatments: rilonacept; anakinra; canakinumab; methotrexate; azathioprine; cyclosporine; intravenous immune globulin (IVIG)
Additional eligibility criteria will be assessed by the study team during screening.
Study Overview
Duration:
![]() Participants will be enrolled in the study for approximately 26 weeks
Participants will be enrolled in the study for approximately 26 weeks
Study Visits:
A total of 9 clinic visits are scheduled, with up to 5 visits done virtually under specific conditions as outlined in the protocol.
Study Timeline:
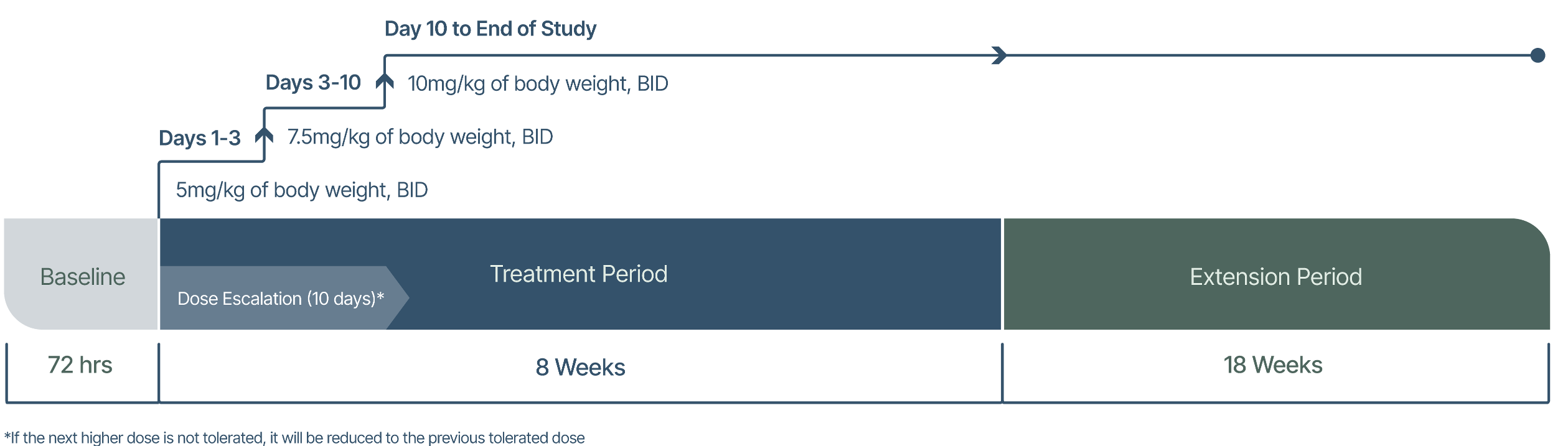
72-hour screening/baseline period
Eligibility will be checked, and baseline assessments completed.
8-week treatment period
CardiolRx™ will be administered orally (via a syringe), with food.
18-week extension period
If there is no contraindication, participants will enter the extension period and continue study treatment for an additional 18 weeks.
During this time, concomitant medications for pericarditis will be weaned under careful supervision.
What is Expected of Study Participants?
The patient will review the detailed information about the study in an informed consent form (ICF) and provide written consent prior to the administration of any study-related assessments, procedures and treatment.
Participants will have to rate their NRS pain score either on paper or via an electronic patient portal.
Participants must not take any prohibited concomitant medications during the study including:
- Digoxin and/or type 1 or 3 antiarrhythmics
- Immunosuppressive therapies including rilonacept, anakinra, canakinumab, methotrexate, azathioprine, cyclosporine, intravenous immunoglobulin
Participants are expected to attend scheduled study visits whether in clinic or virtually (by phone or video call).
Potential Benefits and Risks Study Participants
What are the potential benefits for participants?
- Participants’ overall health and pericarditis symptoms will be closely monitored during the study
- The results of the study could increase the knowledge about the use of cannabidiol in recurrent pericarditis
What are the potential risks for participants?
- Risks associated with study procedures (e.g., risks of blood sampling, irritation due to ECG pads, etc.)
- Potential for hypersensitivity or allergic reaction to the study medicine
- Adverse reactions to the active ingredient in the study medicine may include: diarrhea, fatigue, somnolence, suicidal thoughts (to be monitored during the study), decreased appetite, malaise, skin rash, sleep disorder/insomnia, increased liver enzymes, and infections
- Risks associated with weaning concomitant treatments for recurrent pericarditis
Frequently Asked Questions
Yes. A Phase 1 safety and pharmacokinetic study of single and multiple ascending doses of CardiolRx™ was completed and demonstrated that CardiolRx™ was safe and generally well tolerated at all dose levels, with no serious adverse events reported. In addition to the MAvERIC-Pilot study, CardiolRx™ is also being investigated in the Phase 2 multi-national ARCHER trial to evaluate its safety and tolerability as well as its impact on myocardial recovery, in patients presenting with acute myocarditis (ClinicalTrials.gov Identifier: NCT05180240).
No. However, another form of cannabidiol oral solution (Epidiolex®) is FDA-approved and indicated for the treatment of seizures associated with certain epilepsy syndromes.
CardiolRx™ is pharmaceutically manufactured under Current Good Manufacturing Practice (cGMP), contains high-concentration (100 mg/mL) cannabidiol, and does not contain delta-9 THC (not detected at limit of detection 5 parts per million).
The study medication and all tests and procedures required by the study are provided at no cost to study participants. The costs of other medications, treatments, and procedures used independently of the MAvERIC-Pilot study are not covered by the Sponsor.
Participants will not be paid for being in this study, but will be reimbursed for reasonable expenses (e.g., travel, parking, meals) arising from outpatient visits.
Cardiol Therapeutics, Inc. Contacts
If you would like more information about the study please contact:
Andrea B Parker, PhD
Sr. Director of Clinical Operations
Email: andrea.parker@cardiolrx.com
Andrew Hamer, MBChB
Chief Medical Officer and Head of R&D
Email: andrew.hamer@cardiolrx.com